ISO 13485 is an internationally recognized quality management system (QMS) standard for medical device manufacturers. It provides a framework for Companies to develop a quality management system that meets the needs of customers and relevant regulatory requirements. ISO 13485 Certification in Singapore is intended to be used by organizations involved in the development, manufacturing, installation, and maintenance of medical devices, as well as services related to these industries. It is also appropriate for any business that must demonstrate its capacity to supply medical devices and related services that meet customer and regulatory standards on a consistent basis.
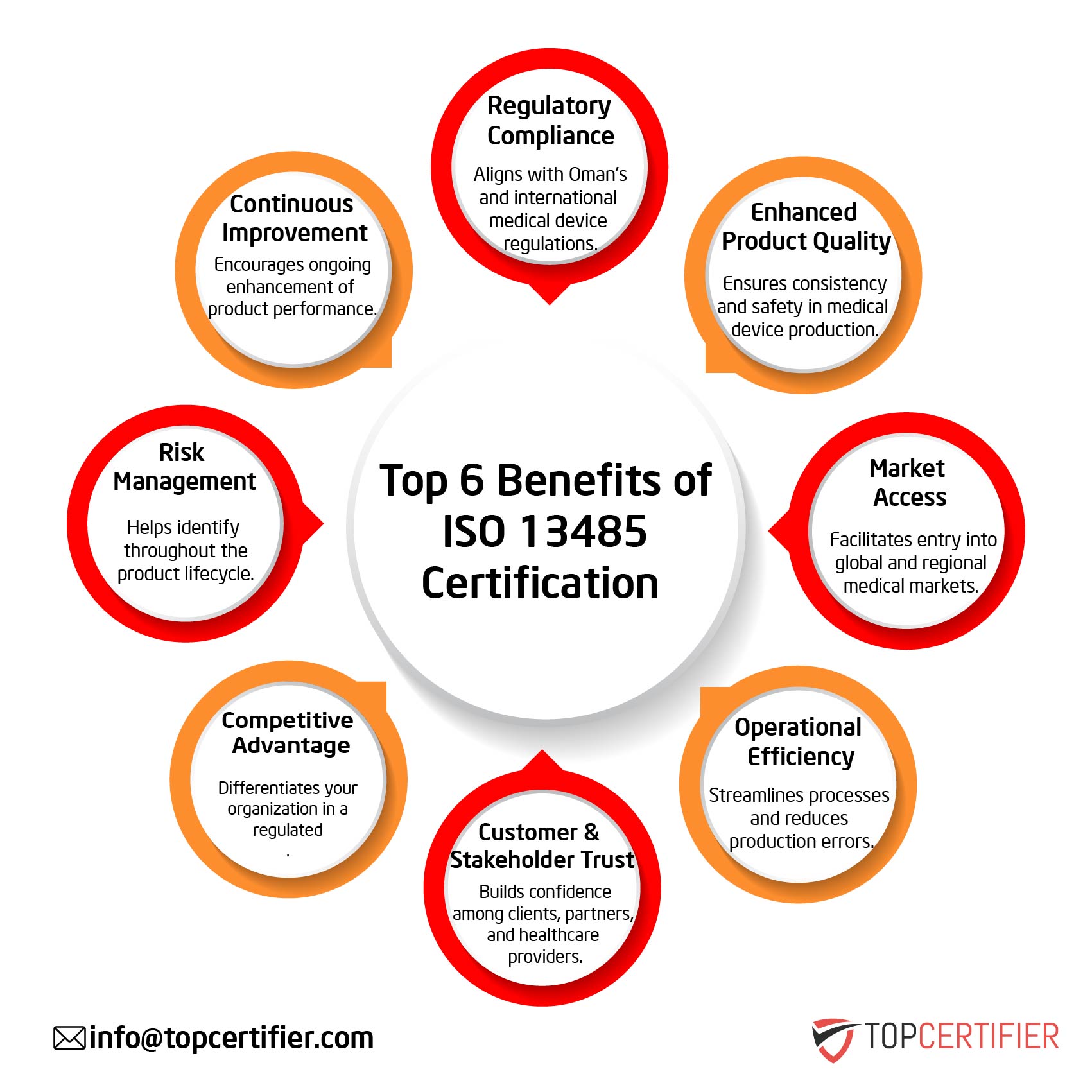
The benefits of ISO 13485 certification in Singapore can be found in its provisions for a comprehensive quality management system. The standard contains requirements for all aspects of quality management, including quality planning, quality control, quality assurance, and quality improvement. Additionally, it provides requirements for risk management, product traceability, and post-market surveillance. When implemented correctly, ISO 13485 can help organizations in Singapore to improve their quality management systems, reduce costs, and increase customer satisfaction.
If you are a medical device manufacturer in Singapore, then ISO 13485 certification is a must. Being ISO 13485 Certified demonstrates that your organization is capable of meeting the requirements of national and international regulatory authorities for the design and manufacture of safe and effective medical devices. Achieving ISO 13485 certification can be a complex and lengthy process, but the benefits are well worth the effort. If you are planning to implement ISO 13485 standard in Singapore , TopCertifier can assist your organization.
TopCertifier is a global consulting firm providing business advisory, training, process consultation, and certification services in Singapore. With operations in 30+ countries and successful completion of 12000+ projects across different standards, we are a one-stop solution provider for all your certification needs. TopCertifier offers ISO 13485 Certification Consulting Services in Singapore across all major locations like Jurong East, Kampong Glam, Tampines, Woodlands. Our services for ISO 13485 Certification include Gap Analysis, Documentation, Training, Process improvement solutions, Organize Internal audits and External audits. We understand the local business culture/ necessities in Singapore and focus on practices that could increase your bottom-line rather than just sticking to standard guidelines and support organizations to achieve certification simpler, faster, and affordably. Therefore, TopCertifier is recognised as one of the Best ISO 13485 Certification Consultants in Singapore.